News

Fourth CSHA conference on “ferroptosis”
Ferroptosis is coming back to Japan! We are excited to announce the 4th Cold Spring Harbor Asia (CSHA) meeting on “Iron, Reactive Oxygen Species & Ferroptosis in Life, Death & Disease,” organized by Quan Chen (Nankai University), Marcus Conrad (Helmholtz Zentrum München & Technical University of Munich), Xuejun Jiang (Memorial Sloan Kettering Cancer Center), Hozumi Motohashi (Tohoku University), Brent Stockwell (Columbia University), and Shinya Toyokuni (Nagoya University). The meeting will take place from November 15–20, 2026, at the beautiful Awaji Yumebutai Conference Center in Awaji, Japan. Awaji Island is conveniently located near Kobe and Osaka and is easily accessible by public transportation. Don’t miss this exciting opportunity to stay updated on the latest breakthroughs in ferroptosis reserach and connect with colleagues and friends during Japan’s beautiful autumn season! (Photo courtesy of CSHA).
See also:
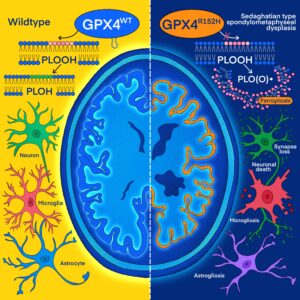
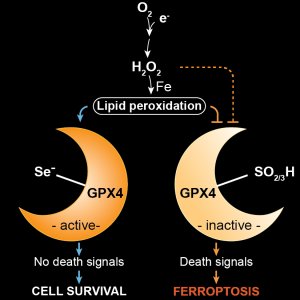
Article published in Cell
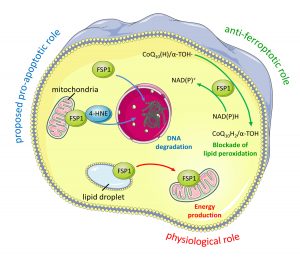
Ferroptosis has long been suspected to contribute to numerous degenerative diseases and to represent a pharmacologically tractable vulnerability in certain cancer states, yet formal proof has largely been lacking. In our current work, we investigated an ultrarare disease, Sedaghatian-type Spondylometaphyseal Dysplasia (SMDS), caused by a biallelic p.R152H mutation in the key antioxidant enzyme glutathione peroxidase 4 (GPX4). Affected children present with severe skeletal abnormalities, widespread developmental delay, and multiple central nervous system defects, including early childhood neurodegeneration. Functional analyses of the R152H GPX4 variant revealed that patient-derived cells, iPSC-derived cortical neurons and forebrain organoids rapidly succumb to cell death upon withdrawal of ferroptosis inhibitors from the culture medium. In-depth structural and orthogonal biochemical analyses demonstrated that the R152H mutation disrupts a previously unrecognized “fin loop”–like structure that protrudes from the GPX4 structure and that enables the enzyme to properly tether to lipid bilayers, thereby preventing harmful lipid peroxidation and ferroptosis. Loss of GPX4 in cortical and cerebellar neurons, or neuron-specific expression of the R152H variant in mice, resulted in widespread neurodegeneration, cortical atrophy, and profound neuroinflammation, features strikingly reminiscent of those observed in patients with Alzheimer’s disease. Notably, a comprehensive neuroproteomic analysis showed that brain tissue from these mice exhibited broad protein dysregulation, including many proteins previously implicated in genome-wide association studies (GWAS) of Alzheimer’s and other neurodegenerative disorders. Together, our findings suggest that neuronal ferroptosis may represent a fundamental mechanism underlying dementia and other neurodegenerative diseases. These insights offer a promising foundation for the development of ferroptosis-targeted therapeutic strategies aimed at alleviating these devastating conditions (Graphical Abstract. Source: Lorenz*, Wahida*, Bostock*, Seibt*, Santos Dias Mourão* et al., Cell 2026).
Original publication:
See also:
Le Monde: Comment meurent les neurones, en cas de maladie neurodégénérative ?
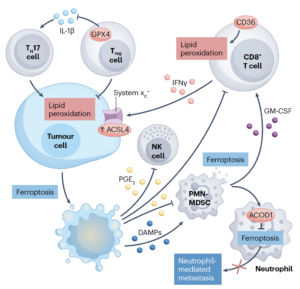
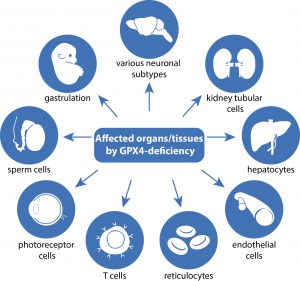
Review article in Nature Reviews Cancer
Over the past decade, ongoing research has revealed that certain cancer states exhibit a marked vulnerability to ferroptosis. Notably, this includes metastatic tumors and therapy-resistant cancer cells, often referred to as “persister” cells, which are thought to contribute to tumor relapse. In this review, we provide a granular and integrated overview of the regulatory mechanisms that govern ferroptosis sensitivity in tumors, highlighting not only tumor-intrinsic factors but also the ways in which ferroptosis shapes the tumor microenvironment and influences tumor immunogenicity. Furthermore, we critically discuss key translational considerations, including promising ferroptosis-related therapeutic targets, potential therapeutic windows, and the pressing need to develop robust ferroptosis-specific biomarkers. Together, these insights aim to support and accelerate the successful implementation of ferroptosis-based strategies into precision clinical oncology (Figure adopted from Wahida & Conrad, Nat Rev Cancer 2025).
Original publication:
Wahida A., Conrad M (2025) Decoding ferroptosis for cancer therapy. Nature Review Cancer

“Highly Cited Researchers” 2025
Tremendously proud to be listed once again as a Clarivate Highly Cited Researcher 2025 — in not just one, but two categories: Biology and Biochemistry and Molecular Biology and Genetics! This marks the sixth consecutive year of receiving this recognition since 2020. Such an achievement is only possible thanks to an amazing, dedicated, and passionate team at Helmholtz Munich (Dr. Bettina Proneth and Dr. Sebastian Doll are also included in the Highly Cited Researcher list!), as well as the numerous inspiring collaborators worldwide with whom I have had the great pleasure of working over the past years. With this extraordinary spirit and motivation, I am confident we will continue to make breakthrough discoveries in the years ahead, all with the ultimate goal of developing new concepts and solutions for some of the most challenging diseases, including tissue ischemia/reperfusion injury, neurodegeneration and cancer (see also Helmholtz Munich).
See also:
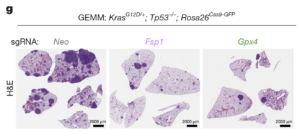
Two articles published in Nature
Thrilled to share that we have contributed to two independent papers published back-to-back in Nature! The first study, led by Thales Papagiannakopoulos at New York University (Wu, Vaughan et al.), demonstrates that targeting ferroptosis suppressor protein 1 (FSP1) alone is sufficient to impair tumor growth in genetically engineered mouse models of lung adenocarcinoma (LUAD). The second study, led by Jessalyn Ubellacker at the Harvard T.H. Chan School of Public Health (Palma et al.), reveals that metastatic melanoma cells develop a unique dependency on FSP1, which can be effectively targeted using small-molecule inhibitors. LUAD and metastatic cancers remain among the leading causes of cancer-related deaths and are notoriously difficult to treat. Excitingly, both studies independently uncover a previously unrecognized and pharmacologically targetable vulnerability in FSP1—something that could not have been predicted from cell culture or organoid studies alone. Previous research across more than 100 tumor cell lines suggested that inhibiting FSP1 was insufficient to trigger cancer cell death, largely due to compensatory mechanisms by other ferroptosis-suppressive systems, especially GPX4. These exciting new findings highlight the critical importance of studying FSP1 in vivo to fully unlock its therapeutic potential. Together, these studies present a compelling case for targeting FSP1 in even the most challenging cancers—especially given that genetic loss of FSP1 is well tolerated in mice. Unlike GPX4, this opens the door to a potentially broad and safe therapeutic window, making FSP1 a truly promising target for future cancer therapies! (Figure adapted from Wu, Vaughan et al., Nature 2025) (see also ” Triggering Cell Death in Metastatic Melanoma and Lung Adenocarcinoma May Pave the Way for New Cancer Treatments”, and National Geographic: “Scientists are figuring out how to make cancer cells self-destruct”)
Original publications:

CDD Jürg Tschopp Award 2025
It is deeply humbling to receive this year’s CDD Jürg Tschopp Award from the European Cell Death Organization. Jürg’s pioneering work not only shaped how we understand the molecular logic of cell death and inflammation, it inspired an entire generation of scientists to think boldly, to challenge dogma, and to connect mechanism to meaning. For me, this award is a milestone along a shared journey that began more than 25 years ago in Munich — one defined by the privilege of working with extraordinary lab members, mentors, and collaborators who make science a collective adventure rather than a solitary pursuit. Thank you to everyone who has shared ideas, experiments, and late nights and very early mornings along the way. This recognition belongs as much to you as to me.
See also:

First international SPP 2306 meeting
Current and former members of the DFG-funded priority programme SPP 2306 gathered from September 30 – October 2, 2025, in Feldkirchen-Westerham, Upper Bavaria, to share the latest discoveries and exciting new developments in the flourishing field of ferroptosis. Thanks to the overwhelmingly positive feedback from all participants, we are confident this was a truly unforgettable event, featuring outstanding scientific talks and poster presentations in an inspiring and highly stimulating environment. A heartfelt thank you goes to all the invited speakers from around the globe who enthusiastically joined us and contributed so much to the success of this meeting. We are already looking forward to the next opportunity to meet again. Hope to see you soon at one of our upcoming events!
See also:

ERC Proof of Concept Grant awarded
We are thrilled to have been awarded a Proof of Concept (PoC) grant by the European Research Council (ERC). This support will help us identify a new lead compound—or potentially a preclinical candidate—for cancer treatment, thereby advancing ferroptosis suppressor protein-1 (FSP1) inhibitors toward preclinical and clinical development. We believe this program holds tremendous potential to deliver a substantial value proposition and open up exciting opportunities for out-licensing or co-development partnerships.
See also:

Paul-Martini-Preis 2025
I am truly honored and humbled to receive the highly prestigious Paul-Martini-Preis 2025. This German medical award is presented annually by the Paul-Martini-Stiftung (Berlin) in recognition of outstanding achievements in clinical pharmacology and drug therapy. Named after Paul Martini, a renowned German physician and clinical pharmacologist who was instrumental in establishing the scientific foundations of modern clinical research in Germany, it is considered one of the most important awards in the country for clinical medical research, particularly in the field of pharmacology. My deepest gratitude goes to the outstanding team at the Conrad Lab, our numerous national and international collaborators, and the many funding agencies that have supported us over the past decades (Foto – courtesy from Thomas Boehm and the Paul-Martini-Stiftung).
See also:
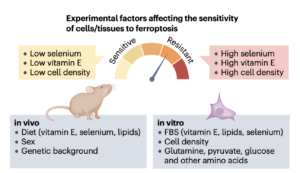
Expert Recommendation in Nature Reviews Molecular Cell Biology
Research into the mechanisms of ferroptosis is soaring due to its relevance to numerous diseases, including ischemia/reperfusion-related complications like stroke and cardiac infarction, neurodegenerative disorders, and cancer. Unlike many other forms of cell death that typically follow specific signalling cues, ferroptosis is impacted by a range of cellular and biochemical processes, including (phospho)lipid metabolism, iron handling, selenium availability, and redox metabolism. Crucially, the stark differences in redox conditions between cell culture systems and whole organisms often hinder the direct translation of in vitro findings to the in vivo context. As a result, well-controlled cellular and animal models, along with rigorously validated tools—such as small-molecule compounds, sgRNAs, and antibodies—are essential for dissecting the mechanisms underlying ferroptosis. In this Expert Recommendation, we explore best practices, highlight common pitfalls, and outline key experimental tools needed to confidently navigate this dynamic field of cell death research. We hope this guide will contribute to harmonizing methodologies across the field and inspire new exciting discoveries. (Figure adopted from Mishima, Nakamura et al., Nat Rev Mol Cell Biol 2025).
Original publication:

Review article in Physiological Reviews
In our most recent review article, we provide a highly detailed and granular perspective on the intersection of ferroptotic cell death and cellular metabolism. Unlike many other forms of cell death, ferroptosis is intricately linked to multiple metabolic processes, including (phospho)lipid metabolism, iron homeostasis, the cholesterol pathway, and redox metabolism and signaling. Given this complexity, a comprehensive understanding of metabolic pathways and key regulatory nodes that continuously monitor the process of (phospho)lipid peroxidation is essential when designing novel therapeutic strategies. Such approaches aim either to prevent ferroptosis in the context of (neuro)degenerative diseases or to induce this form of cell death in certain malignancies (Cover adopted from Physiological Reviews, Volume 105, Issue 2, Apr 2025).
Original publication:

Article published in Molecular Cell
The trace element selenium, discovered over 200 years ago, is a vital component of a select group of selenocysteine-containing enzymes, known as selenoproteins. Among these, glutathione peroxidase 4 (GPX4) serves as the primary guardian against ferroptosis. In this study, we reveal a novel role for peroxiredoxin-6 (PRDX6), a member of the thiol-specific antioxidant protein family. In addition to its established phospholipase and hydroperoxidase activities, PRDX6 exhibits a previously unrecognized “moonlighting” function: binding and delivering selenium to selenophosphate synthetase 2 (SEPHS2). SEPHS2 is a critical enzyme that facilitates the biosynthesis of selenocysteine-loaded tRNA by phosphorylating selenide, a process indispensable for the synthesis of selenoproteins, including GPX4. Notably, the loss of PRDX6 expression significantly increased the sensitivity of tumor xenografts to ferroptosis-inducing conditions and severely impaired selenoprotein expression particularly in the brain, highlighting its essential in vivo role. These findings position PRDX6’s selenium trafficking activity as more pivotal in regulating cellular ferroptosis sensitivity than its previously recognized phospholipase and peroxidase functions (Cover adopted from Mol Cell Volume 84, Issue 23, December 5, 2024)(see also “Novel Selenium Carrier Protein Regulates Ferroptosis in Cancer and Brain“).
Original publication:

“Highly Cited Researchers” 2024
Truly honored and absolutely thrilled to be named a Highly Cited Researcher (“Biology and Biochemistry” & “Molecular Biology and Genetics”) for the 5th year in a row! This achievement wouldn’t be possible without an incredible and motivated team, as well as many successful national and international collaborations. A huge thank you to everyone who made this happen!
See also:

Third CSHA conference on “ferroptosis”
The 3rd Cold Spring Harbor Asia (CSHA) conference on “Iron, Reactive Oxygen Species & Ferroptosis in Life, Death & Disease” organized by Drs. Quan Chen (Nankai University, China), Marcus Conrad (Helmholtz Munich, Germany), Xuejun Jiang (Memorial Sloan-Kettering Cancer Center, USA), Hozumi Motohashi (Tohoku University, Japan), Brent Stockwell Columbias University, USA) and Shinya Toyokuni (Nagoya University, Japan) took place from November 03 to 07, 2024, this time in Suzhou, CHINA. As the two previous meetings, it was incredibly fruitful, fostering a dynamic exchange of ideas and offering novel and exciting insights into the field of ferroptosis. It was characterized by engaging discussions and interactive sessions sparking new collaborations, with participants actively sharing knowledge and challenging perspectives. The environment was not only highly stimulating but also socially enriching, as many attendees connected on a personal level, forming new friendships that promise to extend beyond the conference (Foto taken from CSHA).
See also:

Galenus-von-Pergamon-Preis
Thrilled to announce that our work has been awarded the Galenus-von-Pergamon-Preis 2024 in the category Basic Research. The Galenus-von-Pergamon-Preis is a highly prestigious award established in 1985 to recognize and promote outstanding innovations in pharmaceutical research in Germany. Named after the ancient Greek physician „Galen (Galenos) of Pergamon“, this prize is awarded annually and highlights breakthroughs in drug development and clinical pharmacology that have been introduced to the German market (Foto taken from die Aerztezeitung – copyright Marc-Steffen Unger).
See also:

ECDO Honorary Award 2024
It’s an incredible honor to receive this year’s European Cell Death Organisation (ECDO) Honorary Award 🏆 and to join such a distinguished group of cell death pioneers! This achievement was only made possible by the continued dedication and hard work of the talented members of the ConradLab, both current and past, as well as the many amazing collaborators over the last 25 years. I’m deeply grateful to everyone who has been a part of this journey!
See also:
Article published in Nature Structural & Molecular Biology
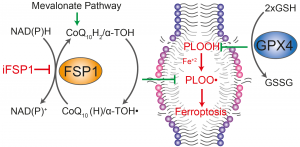
In our current work, we identify and validate the first multi-species inhibitor of ferroptosis suppressor protein-1 (FSP1), called viFSP1 (versatile inhibitor of FSP1). FSP1, along with extra-mitochondrial ubiquinone (CoQ10) or vitamin K, represents one of the key ferroptosis surveillance system in mammals. Since standard therapy-resistant and metastasizing cancers show high vulnerability to ferroptosis, targeting FSP1 might attenuate tumor growth in certain tumor contexts. Moreover, a better understanding of the molecular mechanisms of FSP1 in ferroptosis prevention is of utmost importance for the rationale design of FSP1-based therapies. Using a combination of computational predictions and random mutagenesis approaches, we now unravel not only the mechanism-of-action of viFSP1 and iFSP1, another inhibitor specific for the human enzyme published earlier by us (Doll et al., Nature 2019), but also the enzymatic mechanism of how FSP1 reduces its main substrates CoQ10 and vitamin K, thereby protecting against lipid peroxidation and ferroptosis (Figure taken and modified from Nakamura et al., Nat Struct Mol Biol 2023)(see also “A Rising Star: Inducing Ferroptosis with Innovative Compounds for Cancer Therapy“).
Original publication:

“Highly Cited Researchers” 2023
Delighted to be listed as one of the Highly Cited Researchers again this year. This success is thanks to the tremendous efforts of an outstanding team and fantastic collaborators that I have had the pleasure of working with over the past few years! Clarivate unveiled the 2023 Highly Cited Researchers™ list with 6,849 Highly Cited Researchers in 2023, coming from more than 1,300 institutions in 67 nations and regions. Germany with 336 researchers is ranked fourth after United States, China and United Kingdom.
See also:
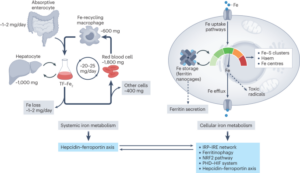
Review article in Nature Reviews Molecular Cell Biology
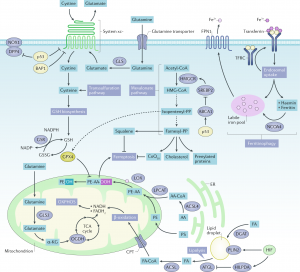
In this comprehensive review article, we provide an up-to-date overview of the multiple roles that iron plays in a variety of cellular and metabolic processes. We highlight how cells handle and transport iron within cells and between organs, and how they use it in the biosynthetic pathways of haem and iron-sulphur cluster-containing proteins. We also discuss the regulatory mechanisms of how cells store and mobilise iron from professional iron storage or release proteins, and the so-called labile iron pool. We emphasise the importance of balanced cellular and tissue iron levels, as disturbances of these are ultimately associated with the development of disease and susceptibility to ferroptosis, a non-apoptotic form of cell death characterised by iron-dependent lipid peroxidation. We believe that a better understanding of the underlying regulatory nodes of iron metabolism is essential for the rational development of new therapeutic options to combat iron-related diseases (Figure adopted from Galy et al., Nat Rev Mol Cell Biol 2023).
Original publication:
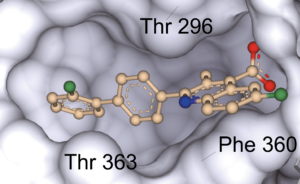
Matters Arising published in Nature
In our recent work, we demonstrate that brequinar (BQR), an established and potent inhibitor of dihydroorotate dehydrogenase (DHODH), sensitizes to ferroptosis via ferroptosis suppressor protein-1 (FSP1) and not via DHODH as previously suggested by Mao et al. (2021). The reason Mao et al. concluded that mitochondrially localised DHODH could be a therapeutic vulnerability in cancer was because of the extremely high concentrations of BQR used throughout, but which also inhibit FSP1, as shown in our recent study. Furthermore, we provide conclusive evidence that it is the short form (alias “cytosolic” or “somatic” form”) of glutathione peroxidase 4 (GPX4) that effectively protects cells from ferroptosis. Our results therefore suggest that DHODH plays only a minor role, if any, in ferroptosis surveillance, while GPX4 and FSP1 remain the major systems for suppressing ferroptosis in mammalian cells. Our study should, however, not discourage ongoing efforts to study DHODH as a valid anticancer target due to its role well-established role in pyrimidine synthesis and hence cell proliferation (Figure taken from Mishima et al., Nature 2023).
Original publication:
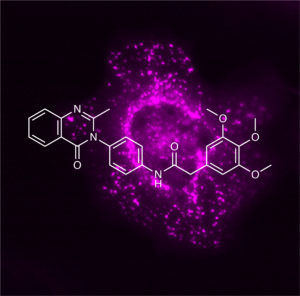
Article published in Nature
In this study, we introduce the small molecule compound class of 3-phenylquinazolinones, termed icFSP1, as the first in vivo active ferroptosis suppressor protein-1 (FSP1)-specific inhibitors. Unlike the first described FSP1 inhibitor iFSP1 (Doll et al., Nature 2019), icFSP1 induces subcellular relocalisation of FSP1 from membranes and phase separation prior to lipid peroxidation and ferroptosis. (Liquid-liquid) phase separation is a physicochemical process similar to the separation of water and oil and is involved in many cellular processes, including cell signalling and transcriptional regulation. In addition, phase separation has also been linked to various pathologies such as neurodegeneration and cancer. We further show that icFSP1-induced phase separation of FSP1 requires different structural elements in FSP1 and that pharmacological treatment of tumour-bearing mice impairs tumour growth. Our results therefore establish a link between the process of phase separation and ferroptosis and should spur future efforts to develop in vivo applicable drugs for the treatment of certain cancers considered to be vulnerable to ferroptosis (Figure taken and modified from Nakamura et al., Nature 2023).
Original publication:
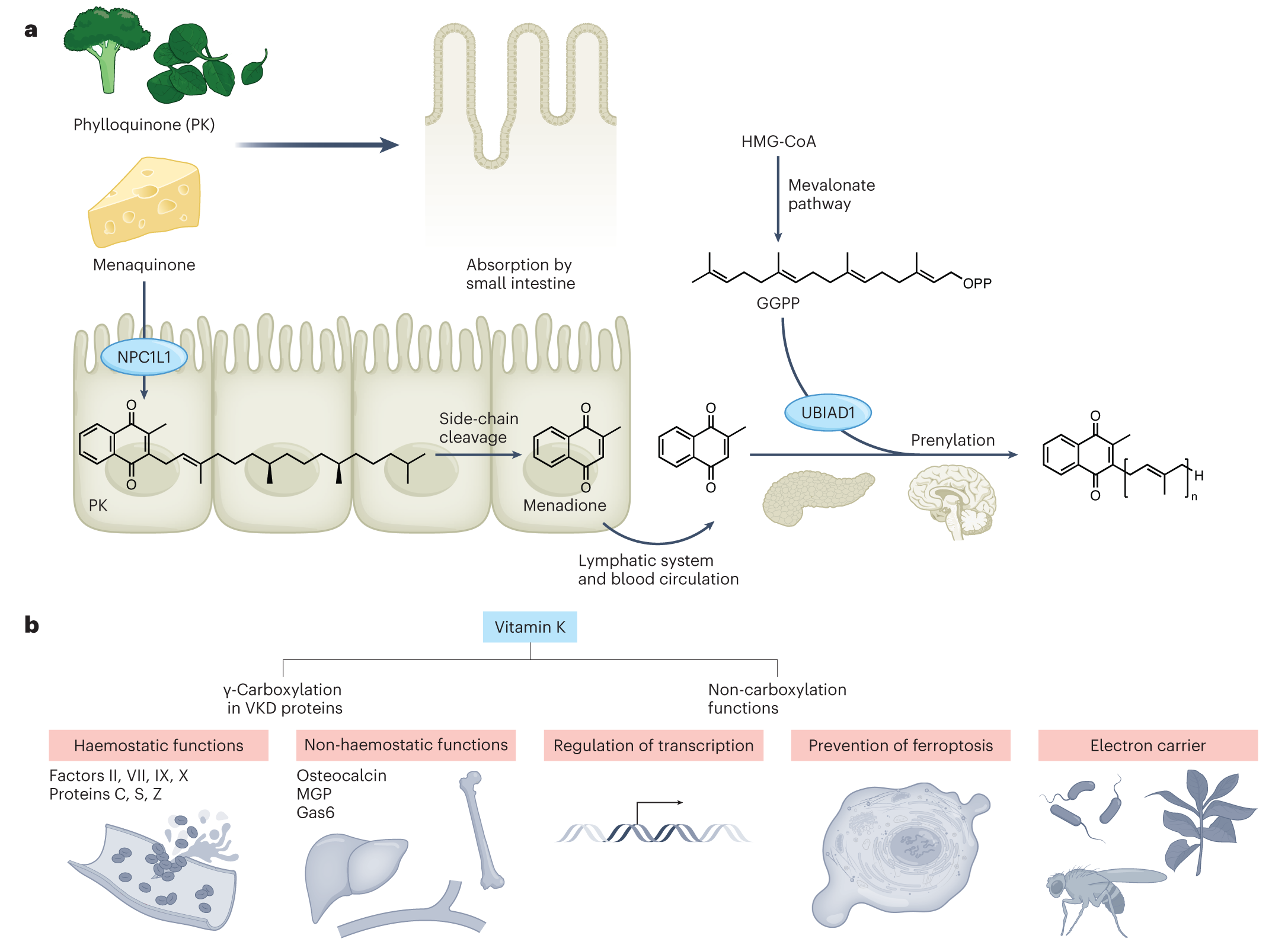
Perspective article in Nature Metabolism
In this perspective article, we provide an overview of the diverse functions of vitamin K (K comes from Koagulation, the German word for coagulation) in physiology and metabolism including blood clotting and bone metabolism. As an essential cofactor of γ-carboxyglutamyl carboxylase in the canonical vitamin K cycle, vitamin K has also vitamin K-independent functions, such as regulation of gene expression. In addition, vitamin K has recently been shown to also act as a potent “anti-ferroptotic” molecule by preventing lipid peroxidation of cell membranes in cultured cells and mice (see Mishima et al., Nature 2022). This non-canonical anti-ferroptotic function, which requires regeneration of oxidized vitamin K by ferroptosis suppressor protein-1 (FSP1) and NAD(P)H, has been termed the “Mishima cycle” to clearly distinguish this function from the canonical function in blood coagulation. Future studies will shed light on whether vitamin K together with FSP1 has similar or even additional functions in organisms that are also highly dependent on vitamin K, such as plants and bacteria (Figure taken from Mishima et al., Nat Metab 2023).
Original publication:

EMBO Workshop “ferroptosis & metabolism”
The first EMBO Workshop entitled “FERROPTOSIS: When metabolism meets cell death” organized by Drs. Marcus Conrad (Helmholtz Munich, DE), José Pedro Friedmann Angeli (University of Würzburg, DE), Maria Fedorova (Technical University of Dresden, DE) and Xuejun Jiang (Memorial Sloan-Kettering Cancer Center, US) took place from April 23 – 27, 2023 in Kloster Seeon, Germany, close to Lake Chiemsee and the Bavarian Alps. We had an excellent lineup of speakers, a huge number of outstanding posters and intense and fruitful discussions. There was clear consensus among the participants that this was a truly exciting and stimulating workshop, sparking new ideas and collaborations.
See also:

New Member of the Hinterzarten Circle of the DFG on Cancer Research
The Deutsche Forschungsgemeinschaft (DFG) has appointed Marcus Conrad as a new member of the DFG’s Hinterzarten Circle on Cancer Research. The Hinterzarten Circle is a roundtable which serves as a regular discussion forum. Membership consists of medical and biological scientists working in the field of cancer research. The annual meeting discusses novel findings in the field of cancer-related basic research and the diagnosis and therapy of malignant tumours. This annual roundtable, held in relative seclusion, aims to encourage intensive discussion between scientists from various disciplines engaged in basic research and those conducting clinical research. The five members of the Hinterzarten Circle are appointed by the DFG for five years.
See also:

“Highly Cited Researchers” 2022
Very honored to see that our work has been recognized again by the broader scientific community. Each year, Clarivate™ identifies the world’s most influential researchers ─ the select few who have been most frequently cited by their peers over the last decade. In 2022, fewer than 7,000, or about 0.1%, of the world’s researchers, in 21 research fields and across multiple fields, have earned this exclusive distinction. Marcus Conrad is among this elite group recognized for your exceptional research influence, demonstrated by the production of multiple highly-cited papers that rank in the top 1% by citations for field and year in the Web of Science™.
See also:

Second CSHA conference on “ferroptosis”
The Cold Spring Harbor Asia (CSHA) conference on “Iron, Reactive Oxygen Species & Ferroptosis in Life, Death & Disease” organized by Drs. Quan Chen (Nankai University, China), Marcus Conrad (Helmholtz Munich, Germany), Xuejun Jiang (Memorial Sloan-Kettering Cancer Center, USA), Hozumi Motohashi (Tohoku University, Japan), Brent Stockwell Columbias University, USA) and Shinya Toyokuni (Nagoya University, Japan) took place from October 10 – October 14, 2022, at Awaji Yumebutai Conference Center, JAPAN. Despite all the hurdles with the COVID pandemics, this second openly accessible conference on ferroptosis was a true success as new findings and developments in the field were intensely discussed among basic scientists, physicians and representatives from pharma/biotech industry.
See also:
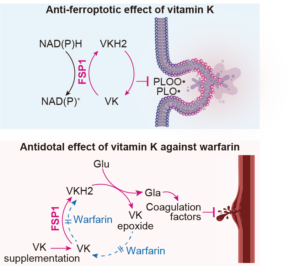
Article published in Nature
In this study, we show that vitamin K is a potent suppressor of ferroptosis by preventing oxidative damage to cellular membranes. So far, vitamin K has been well-known for its role in blood clotting and bone calcification. Its discovery dating almost 100 year ago was awarded with the Nobel Prize in Physiology or Medicine to Henrik Carl Peter Dam and Edward Adelbert Doisy in 1943. We further report that ferroptosis suppressor protein-1 (FSP1) reduces oxidized vitamin K to its hydroquinone form, thus driving a novel non-canonical vitamin K cycle that we tentatively name the “Mishima cycle”. Finally, we demonstrate that FSP1 is the long sought-after warfarin-resistant vitamin K reductase, thus solving one of the last riddles in vitamin K biology. Findings presented in our present work will therefore have immediate implications for both vitamin K and ferroptosis research (Figure adopted from Mishima et al., Nature 2022).

BMBF-funded program on ferroptosis in organ ischemia/reperfusion injury
The newly launched consortium “FERROPath” involves six partners in Germany, located in Munich, Dresden, Regensburg and Essen, who aim to identify and validate common ferroptotic signatures and molecular mechanisms in organs mostly affected by ischemia/reperfusion injury (IRI), such as brain, liver, lung and kidney. IRI occurs when the blood flow to a certain organ is transiently interrupted causing additional damage to the affected tissue due to the generation of high levels of oxygen radicals including lipid peroxidation. IRI is therefore a serious complication, responsible for a variety of clinically important conditions including stroke, acute kidney injury and organ transplantation. As such, understanding the molecular mechanisms and elucidating common molecular signatures of ferroptosis will be of utmost importance for the development of novel therapies based on next generation ferroptosis inhibitors.
See also:

“Highly Cited Researchers” 2021
Delighted to announce that our research of the last years has again been widely appreciated by the broader scientific community, demonstrated by multiple highly-cited papers that rank in the top 1% by citations for field and year in the Web of Science™. Each year, Clarivate™ identifies the world’s most influential researchers ─ the select few who have been most frequently cited by their peers over the last decade. In 2021, 6,602 researchers from more than 70 countries and regions have earned this exclusive distinction (Germany ranks five with 331 scientists).
See also:

Kick-off of the DFG Priority Program SPP 2306
On October 01, we have officially started our DFG (Deutsche Forschungsgemeinschaft) funded Priority Programme “Ferroptosis: from Molecular Basics to Clinical Applications” (SPP 2306) which will fund 25 projects scattered all over Germany for up to six years. The overall goal of the program is to better understand the molecular mechanisms of ferroptosis as the basis for the development of novel therapies based on ferroptosis modulation.
See also:

Review article in Trends in Endocrinology & Metabolism
Here, we highlight the role of lipid metabolizing enzymes, distinct (phospho)lipid species and their oxidative modification as well as relevant metabolic pathways in the context of sensitization of cells toward ferroptosis. We further discuss outstanding questions how lipid peroxidation and related secondary products may contribute to the rupturing of cellular membranes, the final step in ferroptosis execution (Cover art from Trends in Endocrinology & Metabolism. July 2021).
Original publication:

Review article in Nature Reviews Molecular Cell Biology
In our most recent review, we offer a state-of-the-art overview of the key ferroptosis regulating systems and highlight which metabolic processes and signaling pathways ultimately impinge on the cells’ sensitivity towards ferroptosis. Equally important, we provide a guideline (the “DO’S AND DON’TS” in ferroptosis research) about the strengths and limitations of the tools that have been instrumental in delineating the molecular mechanisms of ferroptosis in cells and animal models of disease (Cover art from Nature Reviews Molecular Cell Biology. April 2021 volume 22 no. 4).
Original publication:

Advisory board member of Molecular Cell
Happy to announce that Marcus Conrad was nominated as member of the Advisory Board of Molecular Cell (a Cell Press journal and companion to Cell), a leading journal that strives to publish the best papers in molecular biology.
See also:
“Highly Cited Researchers” 2020
Happy to announce that our research of the last years has been widely appreciated by the broader scientific community, demonstrated by multiple highly-cited papers that rank in the top 1% by citations for field and year in the Web of Science™. Each year, Clarivate™ identifies the world’s most influential researchers ─ the select few who have been most frequently cited by their peers over the last decade. In 2020, fewer than 6,200, or about 0.1%, of the world’s researchers, in 21 research fields and across multiple fields, have earned this exclusive distinction.
See also:
Review article in Cell Metabolism
In our most recent article, we provide a comprehensive review of the molecular mechanisms of ferroptosis with special focus on metabolic pathways that ultimately impinge on the cells’ susceptibility toward ferroptosis. Due to the soaring numbers of publications in this rapidly evolving topic, we deemed a state-of-the-art review appropriate and timely in order to get a quick glance of this exciting field of research with ultimate implication for numerous diseases (Figure adopted from Zheng and Conrad, Cell Metab 2020).
Original publication:
Zheng, J. & Conrad, M. (2020): The Metabolic Underpinnings of Ferroptosis, Cell Metabolism
ERC Advanced Grant awarded
We are happy to announce that our research on the molecular mechanisms of ferroptosis will be funded by the European Research Council (ERC) for the next five years. The mission of ERC is to support the highest quality research in Europe through competitive funding across all fields purely based on scientific excellence. With this funding we strive to provide detailed mechanistic insights in the regulation of ferroptosis, knowledge that will aid in the design of future targeted therapies based on ferroptosis modulation.
See also:
Spotlight for ERC Advanced Grant awardees Magdalena Götz and Marcus Conrad
Perspective article in Nature Chemical Biology
In this Perspective article, we discuss the chemistry of lipid peroxidation, the oxidative modification of lipid bilayers and hallmark of ferroptosis, and how it can be triggered by enzymatic and more importantly by non-enzymatic routes in cells. Ferroptosis is emerging as one of the key cell death pathways contributing to the initial loss of cells and to organ failure in numerous degenerative diseases. On the contrary, activating this form of cell death holds the great potential to fight difficult to treat cancers. Therefore, an in-depth understanding of the molecular underpinnings of lipid peroxidation is mandatory as it offers exciting and still largely untapped opportunities for novel pharmacological interventions.
Original publication:
Conrad, M. & Pratt, D.A. (2019): The chemical basis of ferroptosis. Nature Chemical Biology
Article published in Nature
In this article, we introduce the second ferroptosis preventing system, called ferroptosis suppressor protein-1 (FSP1), that acts independently of the canonical cysteine/glutathione/glutathione peroxidase 4 (GPX4) axis. Unlike GPX4 that protects against detrimental lipid peroxidation by reducing hydroperoxides in lipid bilayers to their corresponding alcohols in a glutathione dependent manner, FSP1 regenerates extramitochondrial ubiquinone that prevents lipid autoxidation by reducing lipid radical species. Since FSP1 is expressed in most cancer cell lines, it represents an attractive drug target for anticancer strategies (Figure adopted from Doll et al., Nature 2019).
Original publication:
Doll, S. et al. (2019): FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature
Perspective article in Nature Reviews Cancer
In this Perspective article we summarize the known mechanisms that regulate sensitivity to ferroptosis in cancer cells and how the modulation of metabolic pathways regulating ferroptosis sensitivity might reshape the tumor niche, leading to an immunosuppressive microenvironment that ultimately promotes tumor growth and progression. We further discuss how this metabolic rewiring particularly during dedifferentiation of certain cancer cells leaves a vulnerability towards ferroptosis, which, in turn, may offer unprecedented pharmacological opportunities to eradicate difficult to treat cancers (Figure adopted from Friedmann Angeli et al., Nat Rev Cancer 2019).
Original publication:
First conference on “ferroptosis” open to the public
The Cold Spring Harbor Asia (CSHA) conference on “Iron, Reactive Oxygen Species & Ferroptosis in Life, Death & Disease” organized by Drs. Quan Chen, Institute of Zoology, CAS, CHINA, Marcus Conrad, Helmholtz Zentrum München, GERMANY, Xuejun Jiang, Memorial Sloan-Kettering Cancer Center, USA, and Brent Stockwell, HHMI/Columbia University, USA, took place in Suzhou, China, from November 26-30, 2018 (https://www.csh-asia.org/2018meetings/ros.html). With more than 200 participants it was a great success as it also brought together many scientists who have made landmark contributions to this emerging and exciting field. It was thus decided to organize a follow-up conference to be announced soon.
Review article in Genes & Development
In our most recent review article we outline the potential similarities and differences concerning the role of lipid peroxidation and associated ferroptosis in different species ranging from mammals, non-mammalian vertebrates, plants, fungi to bacteria (Figure adopted from Conrad et al., Genes Dev 2018).
Original publication:
Article published in Cell
Exactly 200 years ago, the Swedish scientist Jöns Jacob Berzelius discovered the trace element selenium, who named it after the Greek goddess of the moon Selene. The ferroptosis regulator GPX4 is one of distinct 25 selenoproteins in man with selenium in form of selenocysteine in its active site. In our present work published in Cell we now show that selenium utilization of GPX4 provides full resistance to peroxide-mediated enzyme inactivation and associated ferroptotic cell death. As mammals and other vertebrates express selenium-containing GPX4, the exploitation of a rich set of polyunsaturated fatty acids incorporated in membranes (which are inherently prone to oxidative damage) afforded the development of complex brains (Graphical Abstract. Source: Ingold et al., Cell 2018).
Original publication:

Primer article in Cell
A Primer article published in Cell provides a state-of-the-art review about the current understanding of the molecular underpinnings of ferroptotic cell death and its implication in physiological and pathophysiological contexts. It also advises on tools and guidelines for the study of this disease-relevant form of regulated cell death.
Original publication:

Review article in Trends in Pharmacological Sciences
In our most recent review article published in Trends in Pharmacological Sciences we provide a critical overview about what we know today about the molecular mechanisms of ferroptosis, its involvement in diverse (patho)physiological contexts and the development of inhibitors targeting the process of lipid peroxidation.
Original publication:
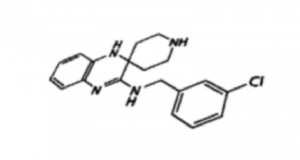
Mechanism-of-action of liproxstatin-1 unveiled
Along with Prof. Derek Pratt, University of Ottawa, we now provide evidence that the potent ferroptosis inhibitors ferrostatin-1 and liproxstatin-1 act as superior radical-trapping antioxidants in lipid bilayers rather than as lipoxygenase inhibitors. We had previously identified liproxstatin-1 as a highly specific, in vivo efficacious ferroptosis inhibitor, being active in in the low nanomolar range with good drug-like properties (see also Friedmann Angeli et al., Nat Cell Biol 2014). In tissues, liproxstatin-1 confers its anti-ferroptotic role by dampening detrimental lipid peroxidation (see also Kagan et al., Nat Chem Biol 2017).
Original publications:
Two articles on ferroptosis in
Nature Chemical Biology
In two publications published back-to-back in the journal Nature Chemical Biology we demonstrate that acyl-CoA synthetase long-chain family member 4 (ACSL4) is a key downstream player in ferroptotic cell death. The mechanism of ACSL4 in the ferroptotic process relies on its function to activate preferably long-chain polyunsaturated fatty acids (PUFA), which when esterified in lipid bilayers (i.e. phosphatidylethanolamines), contribute to the generation of proximate signals for the ferroptotic death program. Moreover, ACSL4 expression was shown to dictate sensitivity versus resistance towards ferroptosis in a subset of triple negative breast cancer cells, indicating that ACSL4 might be developed as a future stratification marker in patients suffering from certain cancers. Hence, our studies add ACSL4 as an essential component in ferroptosis.
Original publications:
Doll, S. et al. (2017): Acsl4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nature Chemical Biology
Kagan, V.E. et al. (2017): Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nature Chemical Biology